Abstract
Background: The costs associated with the treatment of sickle cell disease (SCD) are understudied in low and middle-income countries (LMIC), where resources are scarcer and policy decisions about resource allocation rely on detailed cost data. Few studies have investigated the cost-savings of disease-modifying therapies in SCD in real-world setting. We evaluated the cost of treating SCD-related acute complications and the potential cost-savings of hydroxyurea therapy at HEMORIO, a specialized tertiary hematology center in Brazil.
Methods: The costs (US dollars) of emergency department (ED) and hospitalizations from SCD-related complications between 01.01.2018 and 06.30.2018 were ascertained using absorption and micro-costing approaches. ED costs are presented as cost per each ED event and hospital admission costs are presented as cost per each admission event. ED and admission costs (separated and combined) were determined and compartmentalized into several inputs by fixed and variable categories for all patients over the period of the study [Falk JA et al, Atlas 2001]. The cause of ED or hospital admission visit was verified by a physician and abstracted via medical record review. All hospital admissions were evaluated in the ED. If an ED encounter resulted in an admission, the encounter was counted as an admission event only. The causes related to SCD were grouped and classified according to the discharge diagnosis: 1) VOC (acute pain crisis, priapism or dactylitis); 2) Infection (fever, sepsis, or acute chest syndrome); 3) Anemia exacerbation (acute hemolytic crisis, transient aplastic crisis, or acute splenic sequestration); 4) Chronic organ damage (overt stroke, chronic kidney disease, chronic liver disease or organ failure. Hydroxyurea adherence was estimated by medication possession ratio (MPR) during the study period [Shah N, et al. Health and quality of life outcomes. 2019]. The one-sample proportions test with continuity correction compared the differences in frequency of ED or admissions across discharge diagnoses and the Wilcoxon rank sum test with continuity correction was used to compare cost differences across the groups.
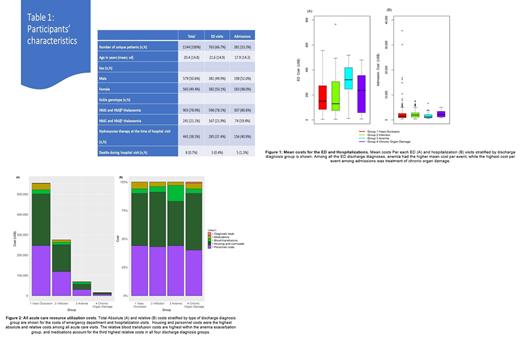
Results: In total, 1144 patients, median age 17 years (range 0-70), 903 (78.9%) with HbSS/HbSβ 0-thalassemia, 441 (38.5%) prescribed hydroxyurea, visited the ED, of whom 381 (33%) were admitted. VOC accounted for 64% of all ED visits and 60% of all admissions (Table 1). Anemia exacerbation was the most expensive reason for ED visit ($321.87/visit), while chronic organ damage carried the highest admission cost ($2,176.40/visit) (Figure 1). Compared with other genotypes, individuals with HbSS/HbSβ 0-thalassemia were admitted more often (79% versus 21%, p<0.0001), and their admission costs were higher ($1,677.18 versus $1,224.47/visit, p=0.0001). Antibiotics and analgesics accounted for 43% and 42% of the total ED costs, respectively, while housing accounted for 46% of the total admission costs (Figure 2). In a regression tree analysis, costs of ED visits were lower among HbSS/HbSβ 0-thalassemia individuals with hydroxyurea MPR ≥65% compared with those with MPR<65% or untreated ($182.46 versus $98.16/visit, p=0.0007). No difference in admission costs were observed relative to hydroxyurea use.
Discussion: It is estimated that between 60,000 to 100,000 individuals live with SCD in Brazil today [Lobo CL, et al. Pediatric blood & cancer. 2014]. In a LMIC hematology-specialized center, VOCs accounted for most acute visits from patients with SCD, but costs were highest due to anemia exacerbation. Analgesics, antibiotics, and housing drove most expenses. Our results confirm the lower acute health care resource utilization with hydroxyurea therapy (fewer ED visits and admissions among those prescribed this medication). Hydroxyurea therapy reduced ED costs among individuals with HbSS/HbSβ 0-thalassemia, however, was dependent on adherence level. A cost analysis of individuals with SCD has never been performed in Brazil previously. Our study will facilitate appropriate planning of allocation of funds and development of health policies for individuals with SCD and may serve as the benchmark against which new SCD disease-modifying therapies may be compared for cost-effectiveness and cost-savings estimation in LMIC, such as Brazil.
Lobo: Novartis: Consultancy. Hankins: Global Blood Therapeutics: Consultancy; UpToDate: Consultancy; Vindico Medical Education: Consultancy; Bluebird Bio: Consultancy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal